“Application of innovative technological approaches, grounded in the principles of sustainability and digitalization, for the development of a new platform of three-dimensional printed personalized drug-delivery systems for non-steroidal anti-inflammatory drugs”

Objective
AT A GLANCE
AIMS
METHODOLOGY
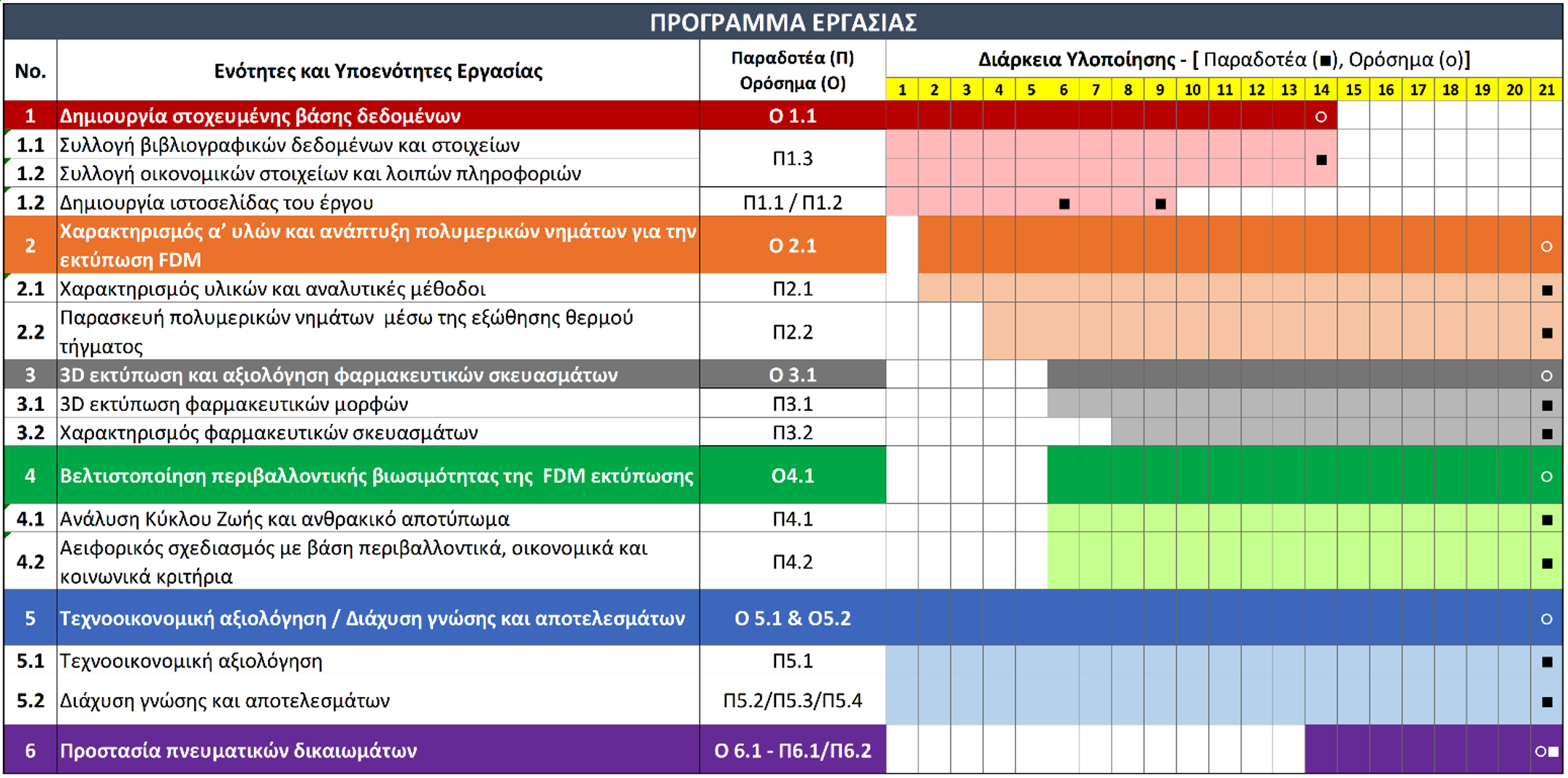
WORK PACKAGES
PARTNERS
At a glance...
3D-SustainDrugs
2
Research partners
2
COMPANIES
21
MONTHS
6
WORKPACKAGES
15
DELIVERABLES
Aims
3D-SustainDrugs
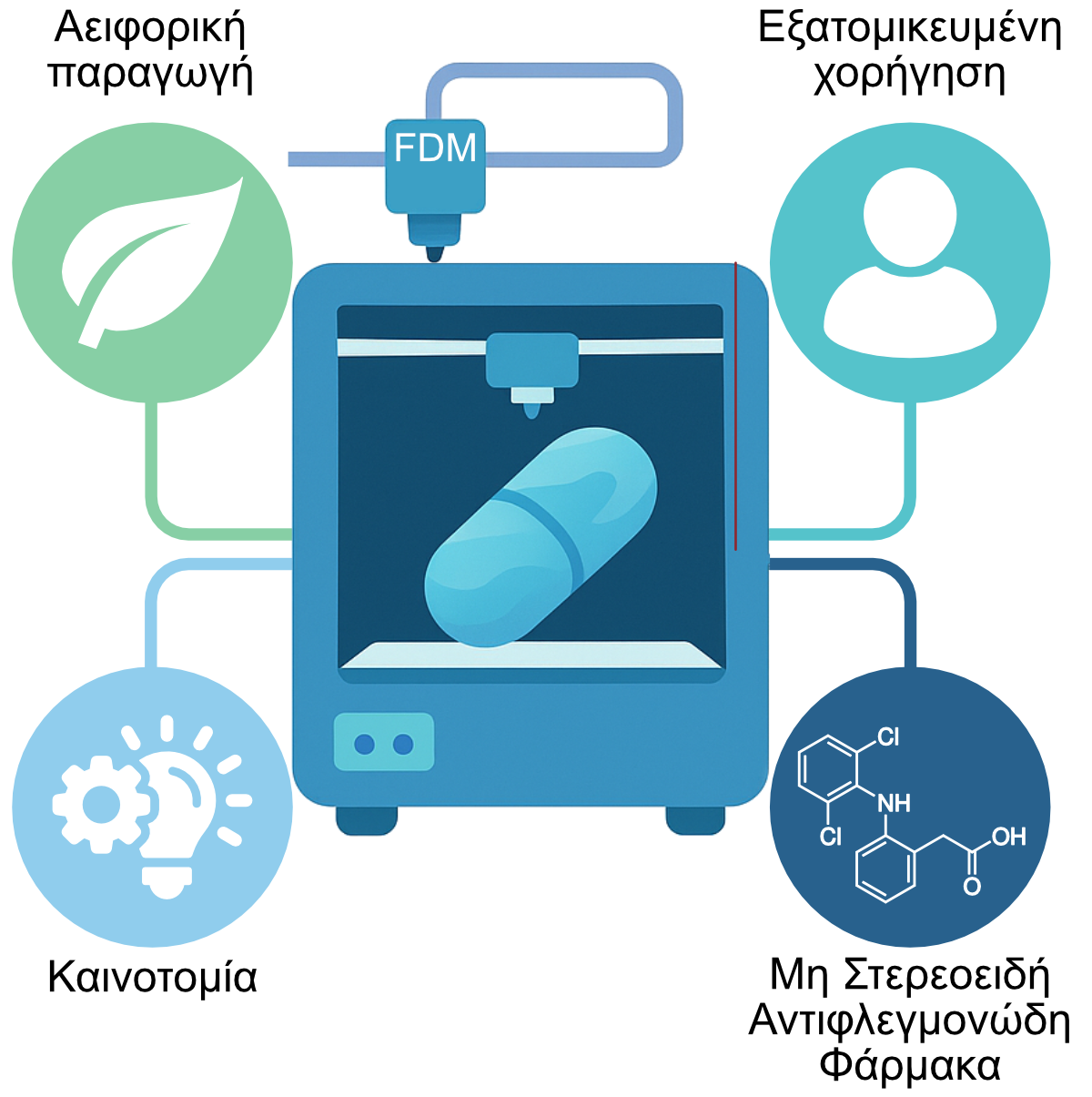
The 3D-SustainDrugs project aims to leverage Fused Deposition Modeling (FDM) 3D printing for the development and production of personalized formulations of non-steroidal anti-inflammatory drugs (NSAIDs), with a strong focus on sustainability and technological innovation. The project seeks to create new pharmaceutical dosage forms through the use of innovative polymeric filaments, produced via environmentally friendly processes.
At the same time, the project aims to reduce the environmental footprint of the manufacturing process, optimize energy efficiency, promote personalized pharmacotherapy, and assess the socio-economic implications of adopting this technology. The ultimate goal is to strengthen the competitiveness of the Greek pharmaceutical industry by enabling the transition to a new, sustainable, and digitally controlled production model aligned with the principles of Industry 5.0.
the main targets of the 3D-SustainDrugs project are:
- The development of innovative technological approaches aimed at adopting sustainable and environmentally responsible practices during the 3D printing of pharmaceutical dosage forms..
- The optimization of electrical energy consumption and CO₂ emissions during FDM printing.
- The introduction of pharmaceutical formulation development and processing into the era of Industry 5.0.
- The development of a new platform of pharmaceutical formulations of non-steroidal anti-inflammatory drugs using FDM 3D printing.
Methodology
3D-SustainDrugs
The project methodology has been designed in such a way as to ensure the successful fulfilment of all technical, business, and social objectives set by 3D-SustainDrugs. Its implementation is based on close interdisciplinary collaboration between two universities (AUTH and IHU), involving research teams from two distinct laboratories: (1) the Laboratory of Pharmaceutical Technology of the School of Pharmacy at AUTH, and (2) the Sustainability Engineering Laboratory and one department (the Department of Supply Chain Management at IHU). Additionally, two companies (KAFSIS and MEDELLA), both possessing significant experience in the development of innovative products/services and in collaborative research projects, participate in the consortium.
The 3D-SustainDrugs partners will work in close cooperation, following the selected methodology, to leverage the existing knowledge, experience, and skills required to achieve the necessary progress and address specific technical challenges. This approach will enable the sustainable and economically viable development of new 3D-printed pharmaceutical formulations for the delivery of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
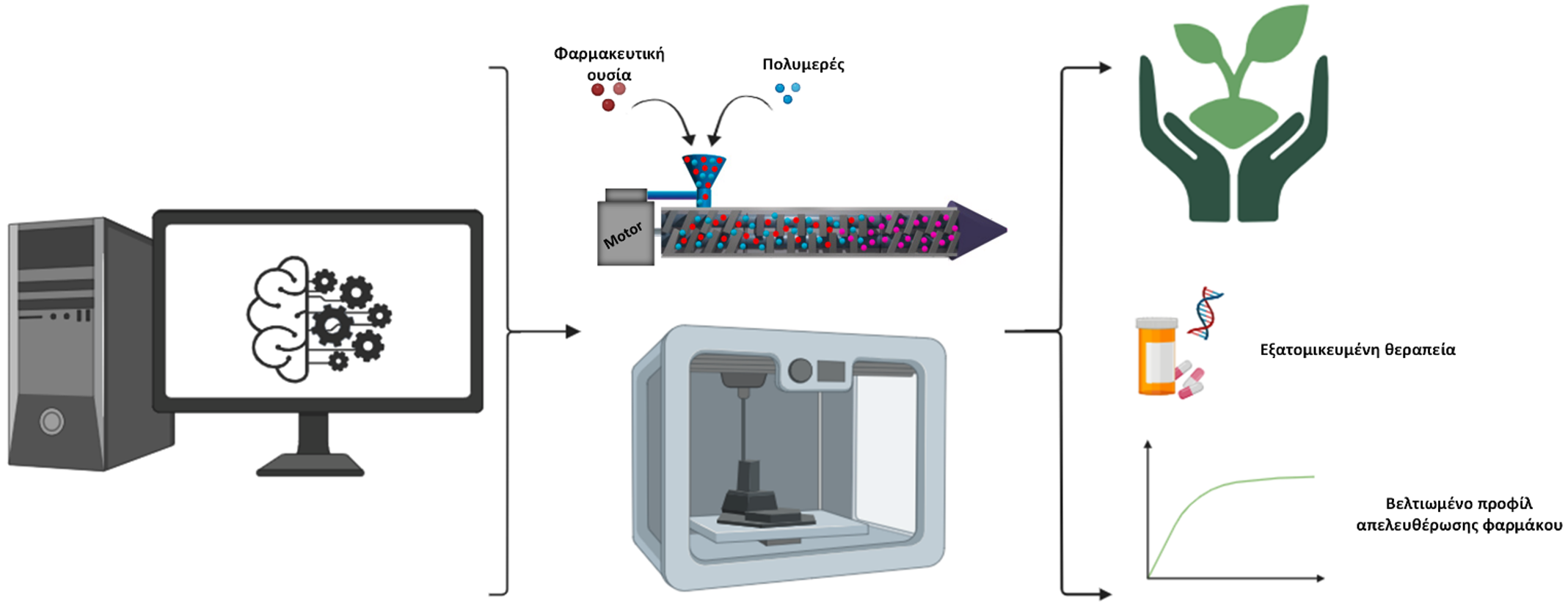
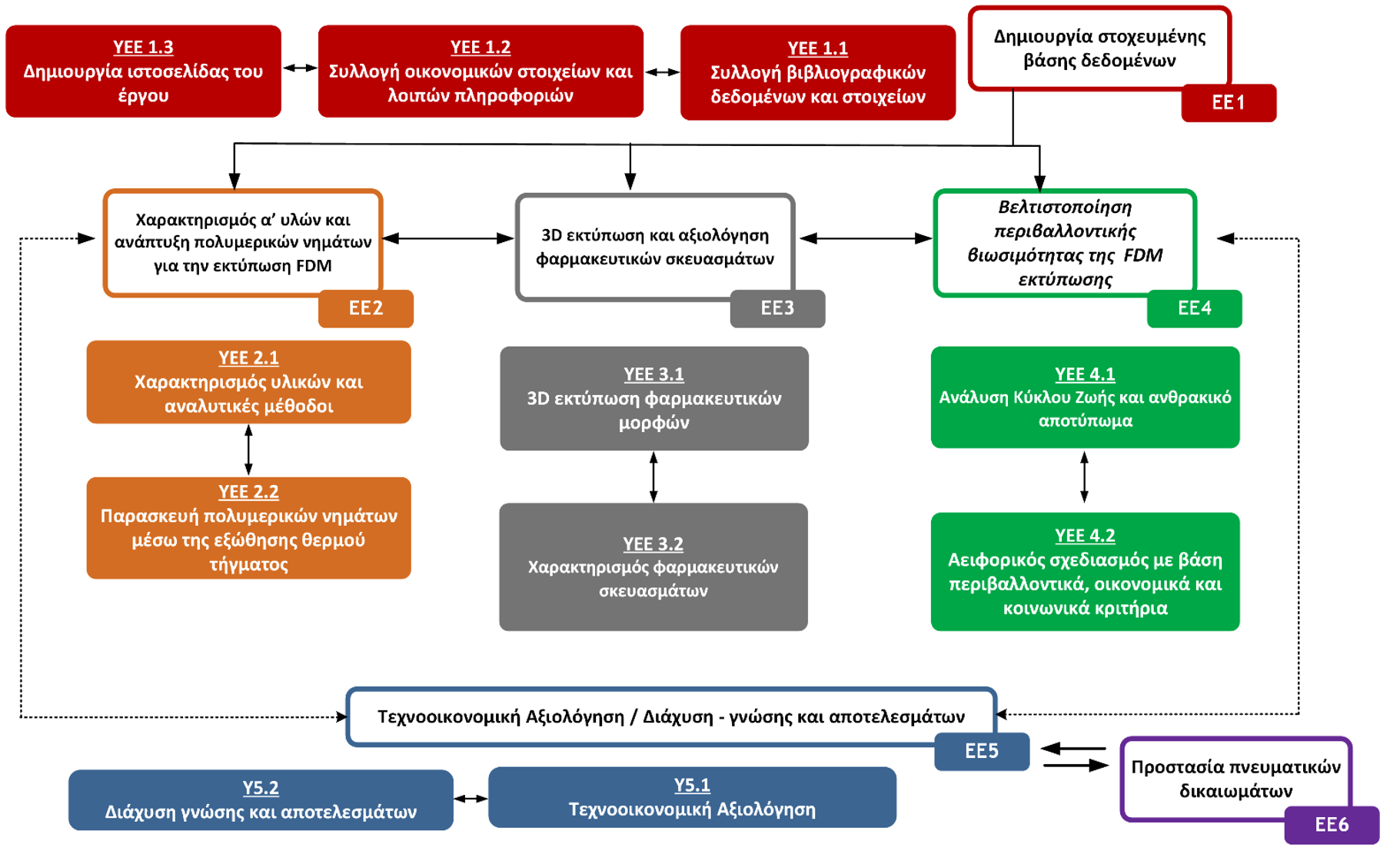
Schematic representation of the 3D-SustainDrugs project methodology.
The adoption of sustainable and environmentally responsible practices in the development of pharmaceutical formulations based on emerging technologies in the Health sector represents a rapidly evolving scientific field with intense research activity, offering significant economic, environmental, and social incentives to all stakeholders. Within this framework, the 3D-SustainDrugs project aims to apply innovative technological approaches, grounded in the principles of sustainability and digitalization, for the development of a new platform of three-dimensionally printed personalized delivery systems for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
For this purpose, the project will first develop a complete, targeted, and integrated database that will include all information necessary for the execution of the project, and which will be shared and continuously enriched with data from all participating partners. This database will serve as the main tool for evaluating and exploiting the results generated throughout the project.
Subsequently, various polymeric filaments will be produced through hot-melt extrusion, which will serve as feedstock for the FDM printer in the fabrication of NSAID drug-delivery systems (DDS). The pharmaceutical substance to be incorporated into the DDS is indomethacin, classified as a Class II drug in the Biopharmaceutics Classification System (BCS), characterized by low solubility in gastrointestinal fluids.
The filaments produced will be studied and evaluated with respect to their characteristics and properties (physical state, formation of molecular interactions, drug content, etc.), and the optimal system will be selected for the development of DDS using FDM 3D printing.
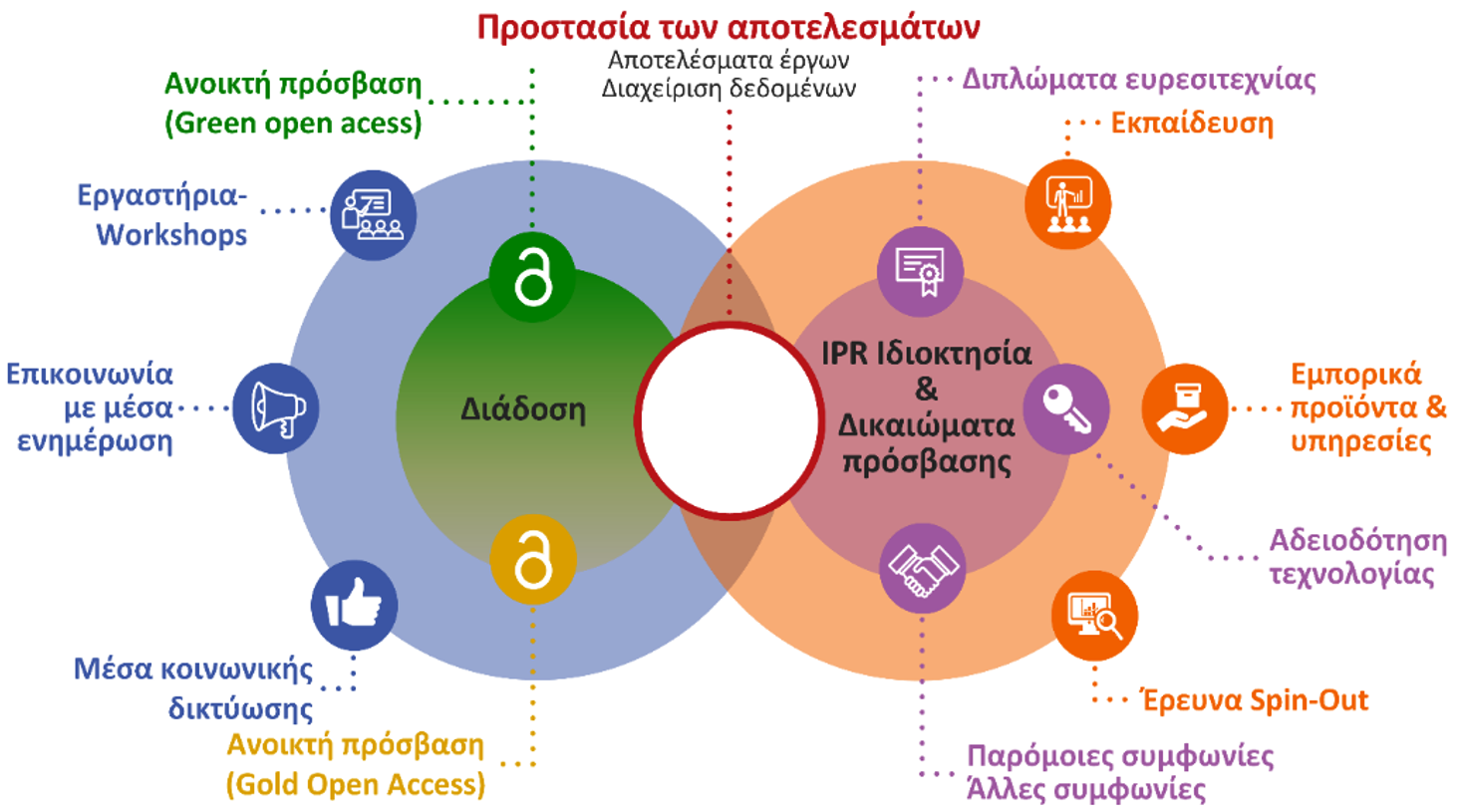
Strategy for managing the results of the 3D-SustainDrugs project
The optimal carrier–plasticizer combination will be the one that yields filaments with acceptable printability, high drug encapsulation efficiency, and strong molecular interactions between the components, while also taking into account the environmental footprint of the process. Subsequently, digital models of the pharmaceutical formulations will be designed, featuring different geometries (for example, tablets of various shapes or torus-shaped forms). The critical printing parameters will then be determined (such as layer thickness, printing speed and temperature, build-plate temperature, infill percentage, etc.), based on the properties and characteristics of the previously prepared filaments.
This will be followed by the 3D printing of the pharmaceutical forms, after which their solid-state properties will be assessed to confirm the successful development of the drug-delivery systems (DDS). Their thermal and physicochemical properties will also be evaluated using appropriate analytical techniques. In vitro dissolution studies will be conducted in suitable aqueous media that simulate gastrointestinal conditions, in order to examine the release profile of the active ingredient from the developed pharmaceutical systems and to verify the enhancement of its solubility through the use of DDS.
In parallel, at every stage of formulation development, the project will implement a holistic “green development” approach, identifying the conditions (such as production-process configuration, selection of raw materials, etc.) that minimize the environmental footprint of the process, and of the resulting pharmaceutical products, within the framework of sustainable development and the circular economy.
Work Packages
3D-SustainDrugs
Creation of a targeted database
Collection and evaluation of the necessary data in order to define the key research, development, and design parameters of the methodologies to be investigated across all phases of the project, as well as the required tools for informing and engaging the relevant stakeholders.
Optimization of the environmental sustainability of FDM printing
(a) Technical analysis of the input flows of energy and broader environmental resources, intermediate flows, and output flows across the entire life cycle of the pharmaceutical formulations produced.
(b) Quantitative assessment of the impacts and environmental burdens arising from the management of the above resources, along with the estimation of the carbon and environmental footprint of the innovative technology under development.
Characterization of raw materials and development of polymeric filaments for FDM printing
Characterization of raw materials and the development and evaluation of FDM printing filaments using appropriate polymeric carriers
Techno-economic assessment / Dissemination of knowledge and results.
Techno-economic evaluation of the product and the development of an appropriate business plan for the exploitation of the results and innovations. Actions for the dissemination of the project’s knowledge and outcomes.
3D printing and evaluation of pharmaceutical formulations
3D printing of the pharmaceutical dosage forms and characterization of their physicochemical properties and in vitro performance
Protection of intellectual property rights
Acquisition and protection of intellectual property rights, e.g., through the granting of patents, for innovations created during the course of the project
Partners
3D-SustainDrugs

Aristotle University of Thessaloniki
Laboratory of Pharmaceutical Technology
Laboratory of Sustainability Engineering
The project “Sustainable development of three-dimensionally printed medicines” (Project Code: ΥΠ3ΤΑ-0560854) is implemented under the Action “Research Excellence Partnerships – REP” and is co-funded by the European Recovery and Resilience Facility “Greece 2.0” and National resources.